A Vision for Internal Light-Based Therapy
The Weber Medical Endolight was born from a bold idea: to bring the power of light-based therapy deep into the bodynon-invasively and with clinical precision. Working in close collaboration with Weber Medical, Eric Djie and the team at Djie Medical translated this vision into a reliable and effective endoluminal phototherapy device.
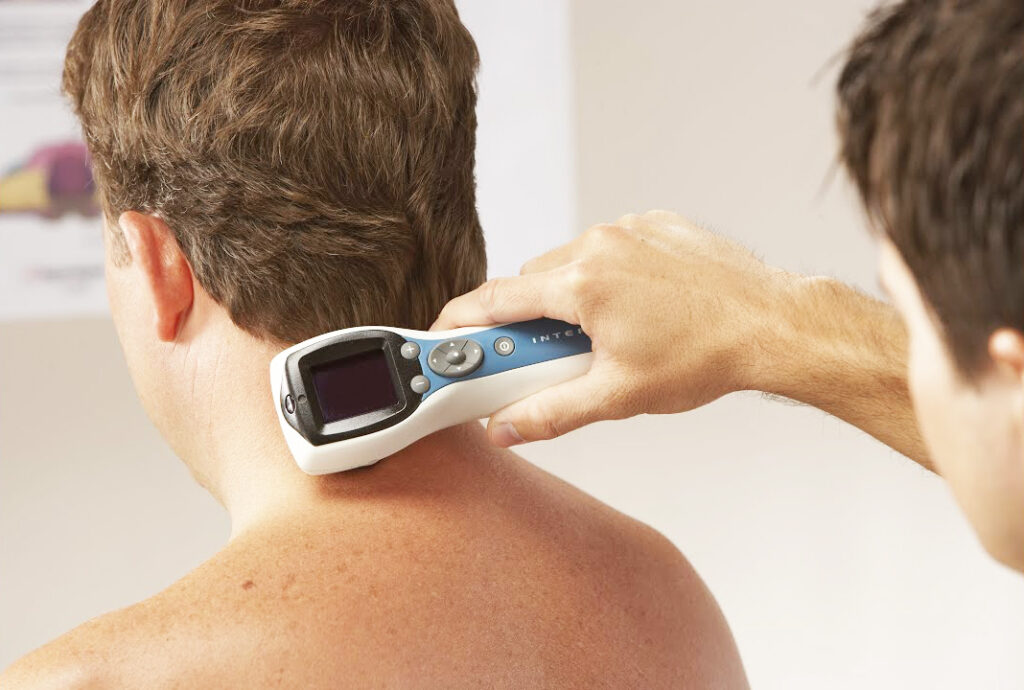
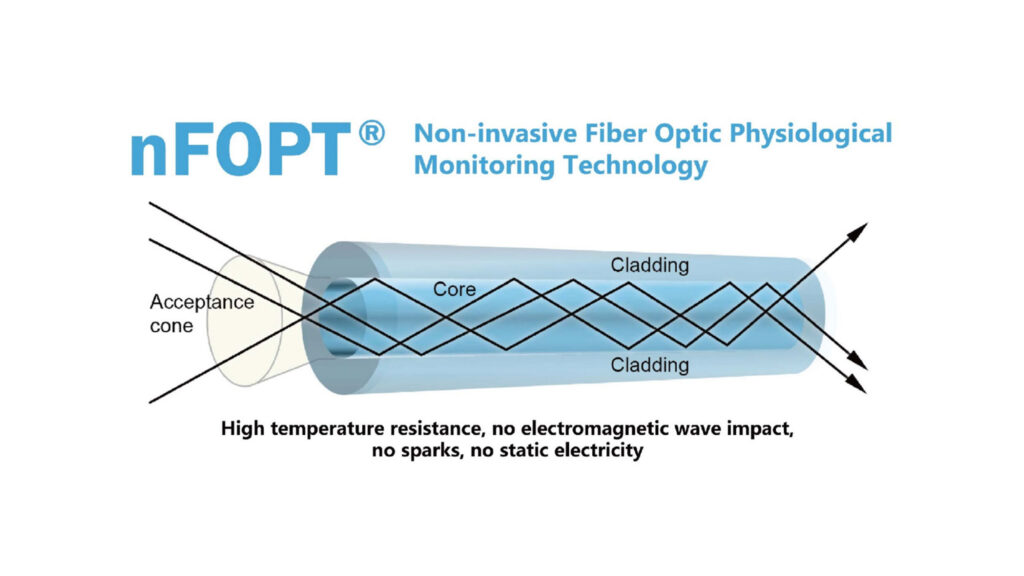
The result is a light-delivery system designed for targeted internal application of photodynamic therapy (PDT) and low-level laser therapy (LLLT). The Endolight enables physicians to reach deep tissues via flexible optical fibers, illuminating treatment sites with controlled wavelengths inside the body for cancer treatment, inflammation modulation, and immune system support.
Our Role: From Concept to Manufacturing

Djie Medical played an end-to-end role in the Endolights development:
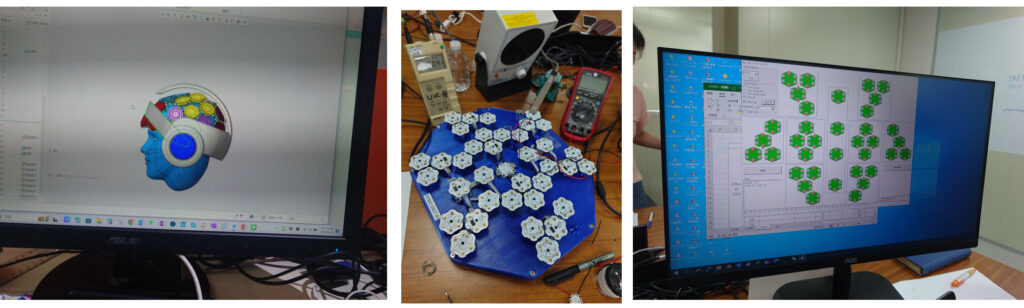
- Feasibility Engineering: Early validation of fiber optics, light dispersion, and medical safety
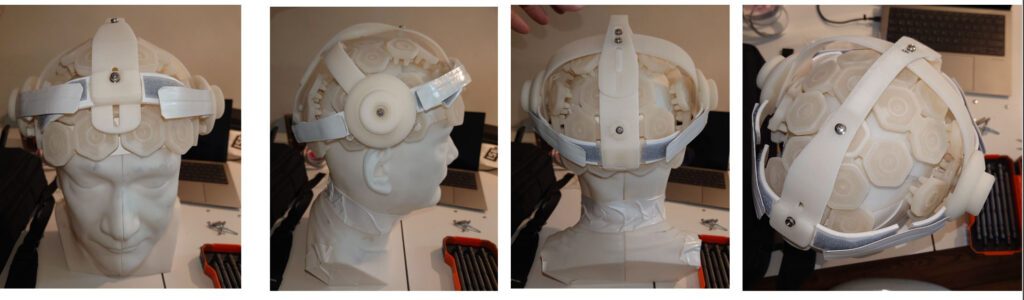
requirements - Mechanical and Industrial Design: Ergonomic, compact form factor suitable for both clinics and
hospital environments - Electrical and Firmware Development: Safe and stable control over multiple wavelengths and
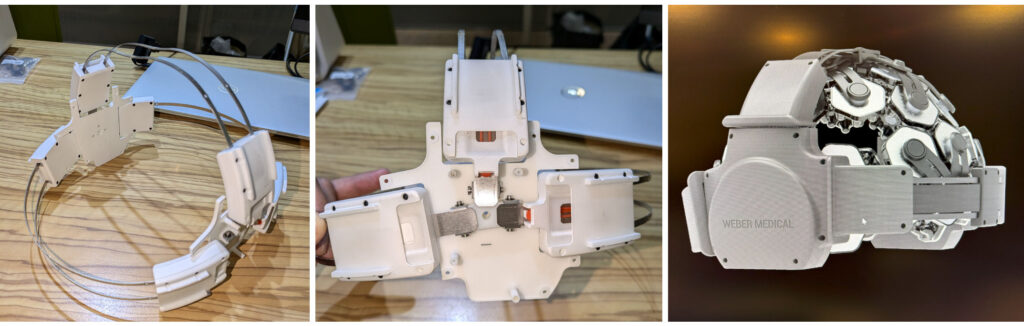
output levels - Prototype Iteration and Testing: Close collaboration with medical users to refine usability and
therapeutic effectiveness - ISO13485-Compliant Manufacturing: Scalable production with full documentation and traceability
This hands-on development cycle ensured the Endolight was not just technically functional but also medically practical and regulatory-ready.

Built on Medical Insight
Eric Djies medical training (Bachelor of Medicine, VUB Brussels) and years of experience in medical product innovation were instrumental in shaping the device:
- Understanding clinical applications like photodynamic tumor treatment and chronic inflammation
- Interpreting clinical research to guide wavelength selection, energy parameters, and usability
features - Engaging with physicians on a professional level to gather feedback and translate it into product
features
This ability to engage with healthcare professionals as both an engineer and medical peer gave the Endolight a unique advantage in both design and acceptance.

Impact and Clinical Use
The Endolight is now a key component of Weber Medical’s global photodynamic therapy portfolio, used in more than 30 countries by clinics focused on:
- Oncology (e.g. PDT with indocyanine green or curcumin)
- Chronic inflammatory conditions
- Lyme disease and stealth pathogen protocols
- Mitochondrial and regenerative therapy
Its compact, flexible light delivery system has expanded the frontiers of non-invasive and systemic phototherapy, offering a high level of control and integration with Weber’s other devices.

From Collaboration to Capability
This case illustrates how Djie Medical transforms complex therapeutic concepts into fully functional medical devices backed by science, designed with care, and built to scale.
The Endolight reflects our commitment to:
- Merging medical insight with precision engineering
- Supporting clinicians with powerful, intuitive tools
- Delivering high-value devices to global health innovators